A simple chemical compound comprises of water and an extra oxygen atom is known as Hydrogen peroxide. It has been firstly recognized in 1818 and is an important covalent peroxide.
Its chemical formula is H2O2
Structure of Hydrogen peroxide
Hydrogen peroxide H2O2 has a non-planar structure. They have two planes with one O-H pair on each plane. The angle between both the planes is 90.2° and the length of O-O bond is 145.8pm and O-H bond length is 98.8pm
As per the valence bond theory, both the oxygen atoms are sp hybridized. Both oxygen atoms have two pairs of unbound electrons each. It has an open book structure.
Preparation of Hydrogen Peroxide
Hydrogen peroxide was industrially prepared by hydrolysis of ammonium peroxydisulphate
- It can be prepared in the laboratory by the action of dilute acids on sodium peroxide
Na2O2 + H2SO4 → Na2SO4+ H2O2 (30%)
- By the action of dilute acid on barium peroxide
BaO28H2O + H2SO4 → BaSO4+ H2O2 + 8 H2O
- By electrolytic process
Electrolysis of 50% Sulphuric acid followed by vacuum distillation and 30% distillate is hydrogen peroxide. The obtained peroxy- disulphuric acid reacts with water to form hydrogen peroxide.
2H2SO4→ 2H+ + 2 HSO4–
At anode
2HSO4– → H2S2O8 + 2e
At cathode
2H+ + 2e → H2
Properties of Hydrogen peroxide
- In dilute solutions, hydrogen peroxide is blue in colour and colourless in room temperature.
- It is slightly viscous than water due to the hydrogen bond formation.
- It has slightly sharp odour as nitric acid
- It forms a homogenous solution when dissolved in water because it is miscible in water.
- Hydrogen peroxide H2O2 has a non-planar structure.
- The value of its acidity is pKa = 11.75.
Uses of Hydrogen peroxide
- Hydrogen peroxide can be used as an antiseptic for minor wounds. As it is a strong oxidizing agent it can kill germs and inhibit the growth of bacterias
- It is a good bleaching agent due to the oxidizing property.
- It is used to restore the colour of oil paintings which contains lead paints
- It is used in cosmetics and medicinal fields.
- It has been used in the certain water treatment process in order to remove impurities
- It can be used as rocket propellant.
- Most of the toothpaste contains hydrogen peroxide for the whitening property.
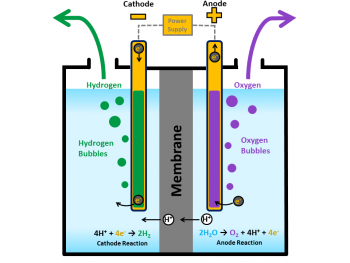
Water Electrolysis
Water electrolysis is the process of splitting water into hydrogen and oxygen in the presence of electricity. The process takes place in an electrolyzer which can be able to produce hydrogen on a small scale.
Working of Water Electrolysis
In this water electrolysis equipment, there will be an anode and cathode which is separated by an electrolyte. Mainly due to the difference in electrolytes, electrolyzers works slightly in a different way.
POLYMER ELECTROLYTE MEMBRANE ELECTROLYZERS
The electrolyte will be solid speciality plastic material.
At anode, water reacts to form oxygen and positively charged hydrogen ions. Electrons will move through an electric circuit and then hydrogen ions move across the PEM to the cathode.
2H2O → O2 + 4H+ + 4e-
At cathode, hydrogen gas is evolved by combining hydrogen with the electrons.
4H+ + 4e- → 2H2
SOLID OXIDE ELECTROLYZERS
The electrolyte used is solid-ceramic that selectively conducts negatively charged oxygen ions.
At the cathode, water is reduced to hydrogen and oxide ions. The oxygen ion passes through the solid ceramic membrane and reacts at the anode to form oxygen gas.
It must operate under 700-800°C to decrease the electrical energy needed to produce hydrogen from water.
Summary
- A simple chemical compound comprises of water and an extra oxygen atom is known as Hydrogen peroxide.
- Hydrogen peroxide H2O2 has a non-planar structure.
- It is a strong reducing agent and used as an antiseptic and bleaching agent
- Water electrolysis is the process of splitting water into hydrogen and oxygen in the presence of electricity.
- Water electrolysis takes place in an electrolyzer. According to the different electrolyte like in fuel cells, it acts slightly differently.
- There are mainly 2 types of electrolyzers according to the different electrolytes present polymer electrolyte membrane electrolyzers and solid oxide electrolyzers.